As a visual learner, chemistry always came naturally to me because it’s a subject where abstract concepts are brought to life through models. Chemical formulas, chemical reactions, orbital diagrams, and electron configurations are among the most iconic and representative models in chemistry because they serve to represent fundamental objects and processes we can’t directly observe. We can leverage the use of models in a more creative, inquiry-based, student-centered way to make learning chemistry easier and more engaging while actually increasing the rigor and raising the expectations for critical thinking.

In this article, I’ll share a dynamic, technology-driven lesson that includes core ideas related to Schrödinger’s model of the atom, orbital diagrams, and electron configurations. The inquiry-based science activity at the center of this lesson emphasizes the science practice related to developing and using models; students construct their own understanding of how Schrödinger’s model of the atom correlates to Aufbau orbital diagrams as they build both models in a free, interactive simulation using trial and error.
If you’re looking to make your chemistry lessons more engaging and student-centered, or if you’re curious about how to structure an inquiry-based lesson, keep reading or watch the video version:
TABLE OF CONTENTS
- How This Electron Configuration Activity Makes Learning Memorable
- What Makes This Electron Configuration Activity Truly Engaging
- A Teacher’s Guide to Leading This Electron Configuration Activity
- Modifying This Electron Configuration Activity For Your Own Classroom
- Making These Models Work Across Your Curriculum
How This Electron Configuration Activity Makes Learning Memorable
Chemistry is a subject where models are foundational to understanding. From early atomic theories to the more modern Schrödinger model, much of what we know stems from scientists’ ability to hypothesize and refine models over time. The Lab In Every Lesson teaching philosophy centers on giving students the same opportunities for exploration! By challenging them with developing and using models of their own, they develop critical thinking skills and learn to evaluate evidence — a core principle of the NGSS science and engineering practices.
This lesson is a quintessential example of inquiry-based learning, combining structure with enough flexibility to maximize student engagement.
Here’s how it unfolds:
Activating and Integrating Prior Knowledge About Atomic Radius
Students begin by reflecting on prior knowledge of the Bohr model and, specifically, the periodic trend related to atomic radius. This activity reminds students that atoms with more orbitals are larger than those with less orbitals. This will be important information to apply as they seek to determine the meaning of the labels in Schrodinger’s model – 1s, 2s, 3s, etc. — while they are developing and using models in the simulation.
Exploration FOR EVIDENCE
Using a dynamic, interactive simulation, students experiment with developing and using orbital diagrams as models to write electron configurations. This student-controlled approach encourages trial and error while fostering perseverance and curiosity.
Evidence Students Extract From The Electron Configuration Activity
The simulation provides real-time feedback, allowing students to evaluate their decisions and adjust their approach. This mirrors the work of scientists who argue from evidence and refine their hypotheses over time.
QUALITATIVE Data-Dependent Analysis
As a means of uncovering core ideas of this content, students evaluate information, communicate their findings, and explain their reasoning for their observations. These skills are integral to inquiry-based learning and prepare students for real-world scientific problem-solving.
Formative Assessment and Skill Practice
We ensure students’ learning experience translates to achievement on assessments by further deconstructing the models they observed so that they can apply them in future practice scenarios.
What Makes This Electron Configuration Activity Truly Engaging
This electron configuration activity is packed with features that make it both highly engaging for students and actionable for teachers. By combining the strengths of technology with inquiry-based learning and NGSS-aligned practices, this lesson connects abstract concepts to tangible understanding.
Here’s how:
Observation-Based Activities
- Students aren’t just observing models—they’re developing and using models. The lesson starts with minimal direction, encouraging exploration and critical thinking.
- Students identify patterns in how orbital diagrams are drawn through trial and error and build the foundation for writing electron configurations. This ramps up the rigor related to learning orbital diagrams and how they relate to Schrödinger’s model.
STUDENT-CENTERED DESIGN
- A dynamic, free web simulation serves as the core tool for discovery. It provides real-time feedback, including hints and corrections, allowing students to test hypotheses and refine their approach.
- The simulation mimics scientific experimentation, helping students experience the iterative nature of science without traditional lab equipment.
Connections to the ngss expectations
- NGSS Standard Alignment: This lesson specifically targets the standard related to structure and properties of matter (HS-PS1-1.) for using the periodic table as a model to predict relative properties of elements based on outermost electron patterns.
- Science and Engineering Practices: Students engage in developing and using models, obtaining and evaluating information, and arguing from evidence. These practices encourage higher-order thinking and align perfectly with NGSS goals.
- Crosscutting Concepts: Students establish patterns as they recognize the repetitive nature of filling orbital diagrams and writing electron configurations. More importantly, though, the lesson highlights systems and system models, demonstrating how electrons in various locations within an atom impact its physical and chemical properties.
PRIOR KNOWLEDGE POTENTIAL FOR AWESOME INFERENCES FROM NEW EVIDENCE
- This is the third lesson in a series that builds foundational knowledge for understanding chemical bonding and reactivity. By this point, students are familiar with the Bohr model of the atom and the periodic trend relating to atomic radius, allowing for a seamless transition into Schrödinger’s more complex model.
TIMELY Teacher INTERVENTION
- The included artifacts—observation outlines and reflection prompts—make it easy for teachers to assess student engagement and understanding.
- This electron configuration activity lesson is ready to implement with minimal prep, thanks to the free simulation and detailed guidance included in the resource.
By emphasizing exploration, evidence-based reasoning, and the power of models, this lesson equips students with the tools to approach chemistry as real scientists do—through discovery and critical thinking.
A Teacher’s Guide to Leading This Electron Configuration Activity
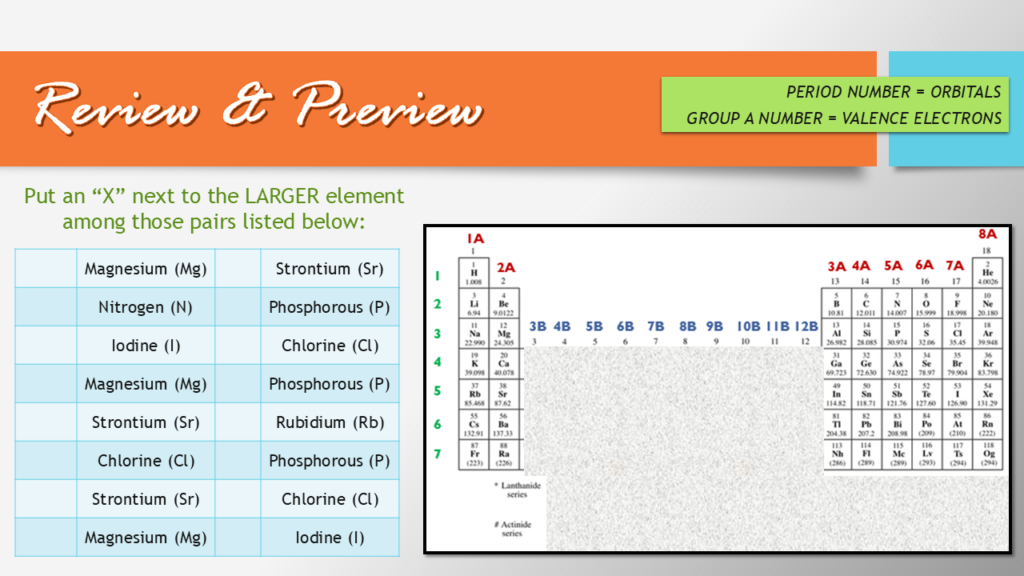
Review and Preview BEFORE ASSIGNING THE ELECTRON CONFIGURATION ACTIVITY
Every lesson in this series on electrons begins with what I call a “review and preview” activity. It’s not a generic word search or crossword puzzle; it’s a meaningful exercise that helps students make connections between what they’ve already learned and the new concepts they’re about to explore. This step is critical—it bridges the gap between lessons and ensures students feel prepared to dive deeper into today’s material.
In this particular lesson, the warm-up focuses on reviewing atomic radius trends. Students compare the sizes of atoms within groups and periods of the periodic table, reinforcing what they learned in the previous lesson. For example, they revisit how atomic radius increases down a group and decreases across a period, analyzing these patterns through eight targeted comparisons. This practice isn’t just about memorizing arrows or trends; it’s about understanding the underlying reasons behind them, like effective nuclear charge.
Establish Reliable Routines
The beauty of this “review and preview” practice is its simplicity. Students complete the warm-up as soon as they enter class—whether it’s picking up a handout at the door in a face-to-face classroom or accessing a digital version in an online environment. It’s quick, taking only about five to ten minutes, but it’s packed with value. Reviewing the atomic radius trends prepares students to connect these ideas to the focus of the upcoming electron configuration activity: how orbital diagrams and electron configurations reflect the structure of an atom.
Activating and Integrating Prior Knowledge
This approach also gives me, as the teacher, a chance to ensure everyone’s on a level playing field, especially in a course that is as cumulative as high school chemistry where so much of the content presented becomes a foundational core idea – reasoning – for future core ideas. By spiraling back to previous topics regularly using a formative assessment-style approach, I can make sure foundational concepts are firmly in place before we move forward.
And it’s not just about looking back—it’s about looking ahead. For instance, during this warm-up, students begin to see how atomic radius relates to the Aufbau principle, where the number before the orbital label (like “2” in 2p) represents an atom’s distance from the nucleus. These subtle connections set the stage for the rest of the lesson, ensuring students are ready to build on what they know and tackle more complex ideas.
Setting Objectives FOR THE ELECTRON CONFIGURATION ACTIVITY
Clear objectives are the backbone of any effective lesson. For this electron configuration activity, I start by outlining learning intentions and success criteria that guide students toward meaningful engagement and measurable outcomes. Inspired by John Hattie’s work on visible learning, I aim to ensure students understand why they’re learning each concept and can evaluate their own progress throughout the lesson, because when students know what they’re working toward and why it matters, they might feel more engaged and confident.
Learning Intentions
- Today, I am learning about Schrödinger’s atomic model so I can talk like a scientist.
This intention emphasizes the disciplinary literacy of chemistry, where understanding symbols, numbers, and terms is as essential as fluency in a new language. - Today, I am learning to use patterns to predict and describe the location and energy of electrons.
While my students have already explored these ideas using the Bohr model, this lesson deepens their understanding by introducing Schrödinger’s more nuanced orbital diagrams and the Aufbau principle.
These intentions highlight that this is an exploratory, discovery-based lesson. Students aren’t just passively absorbing information—they’re actively uncovering the “rules” of orbital diagrams and electron configurations through trial and error.
Success Criteria
To complement the learning intentions, I define clear success criteria that allow students to gauge their progress throughout and following the lesson:
- Activity-Based Success Criteria Related to Developing and Using Models
- Use the simulation to fill orbital diagrams for three atoms (e.g., helium, carbon, and sodium).
- Write corresponding electron configurations for each atom.
- Extract and articulate the guidelines for filling orbitals based on their observations.
- Assessment-Based Success Criteria:
- Demonstrate the ability to fill orbital diagrams and write electron configurations independently.
These criteria are designed to ensure students know exactly what they’re working toward. Even if the lesson spans multiple class periods, success criteria help students [and me!] pinpoint where we left off and where to pick up again.
When preparing objectives like these, it’s important to connect them as directly as possible to the standards you’re using. This electron configuration activity lesson is closely aligned to NGSS standards, particularly the one focused on using the periodic table as a model to predict relative properties of elements based on electron arrangement (HS-PS1-1.).
lEADING sTUDENTS tHROUGH THE eLECTRON cONFIGURATION aCTIVITY TO cOLLECT oBSERVATIONS
At the heart of this lesson is a student-controlled, observation-driven activity where students are developing and using models — specifically, building Schrodinger’s model of the atom while collecting information as to how the orbital diagram of that atom would be filled. This process transforms abstract ideas, like Schrödinger’s model, into engaging experiences that prioritize discovery and critical thinking.
Technology Is More Effective Than Talking
Using a free, web-based simulation, students build a visual representation of Schrödinger’s atomic model. They drag and drop orbitals (1s through 3p) into place, positioning them based on their spatial orientation (e.g., 3px, 3py, 3pz). As students fill these orbitals, the simulation generates corresponding box diagrams with arrows denoting electron spin. This simultaneous display helps students connect the physical layout of orbitals to the symbolic language of orbital diagrams and electron configurations, both developing and using models!
The activity starts small, with helium—a simple two-electron atom. Students progress to more complex elements like carbon, sodium, and argon, gaining repeated practice with the orbital filling rules: the Aufbau principle, Pauli exclusion principle, and Hund’s rule. This gradual progression cements understanding while building confidence. If you’ve grouped students, it also provides an opportunity for a valuable exchange of ideas.
The web tool, itself, provides real-time feedback, offering hints or corrections as students attempt to place electrons in orbitals. This immediate reinforcement encourages trial and error, helping students uncover patterns and rules independently.
Instead of lecturing the guidelines for filling orbital diagrams and relying upon note-taking and rote memorization to lead learning, students build their understanding through the active exploration they engage in while developing and using models.

Observations Reduce Overwhelm To Increase Engagement
This electron configuration activity is not about overwhelming students with terminology or complex tasks right away. It’s also not about presenting a pattern and expecting students to memorize it through drill practice. Instead, it simplifies the process of learning through observation – through developing and using models. Encouraging students to focus on what they see, this low-stakes approach reduces student overwhelm and lowers barriers to engagement. By framing the task as an observational exercise, students can focus on exploring the simulation without the pressure or expectation of immediate mastery.
The language we use in presenting tasks like this electron configuration activity is critical. Directing students to “just observe and tell me what you notice” nurtures a culture of curiosity and exploration. This subtle but critical shift in approach keeps students invested and sets the stage for meaningful learning. Plus, if we imagine the scientific method as a learning taxonomy all its own, observations are the lowest level of that hierarchy and are, therefore, most accessible to every student.
Artifact Outlines Provide Structure
In this lesson, students create detailed artifacts that not only emphasize discovery and serve to document their learning but also provide the foundation for meaningful, evidence-based discussions. The process is intentionally designed to move students from observation to analysis, emphasizing the importance of citing evidence and reasoning from their own data.
As they are developing and using models of the Schrodinger atom in the simulation, students are documenting the arrows and positions that appear in the box diagrams. This outline becomes a living record of their work, connecting the visual representation of atomic orbitals to the symbolic notation of electron configurations.
By both developing and using models independently during both the observation portion of the lesson and the analysis portion of the lesson, students naturally engage with the following key questions:
- What do the numbers in orbital labels indicate?
- How do letters like “s” and “p” relate to orbital shapes?
- Why are there limits on the number of electrons per orbital?
These questions arise organically, and instead of being spoon-fed answers, students uncover the orbital diagram filling rules themselves. This reflective process not only solidifies their understanding of principles like the Aufbau principle, Pauli exclusion principle, and Hund’s rule but also builds confidence in their ability to think critically and solve problems. When repeated with consistency, this approach develops in students a sense of ownership over their learning.
Once students students have finished developing and using models for all three atoms in the simulation, a student-centered way to approach reviewing the outcomes involves having students “teach back” the principles they’ve discovered. Often, their responses are insightful, reflecting the depth of understanding they’ve gained through the activity and the confidence that comes from knowing they discovered these concepts themselves!
Easy-Does-It With Some Differentiation
Depending on the preparedness of your students, this analysis can be heavily guided or entirely student-driven. For advanced learners, you might be able to simply ask them to list what they’ve learned. For students who need more support, you can include prompts to answer specific questions related to the core ideas or success criteria of the lesson.
skill Practice fOLLOWING tHE eLECTRON cONFIGURATION aCTIVITY
The lesson concludes with targeted skill practice to ensure students can apply their learning. This practice bridges the gap between discovery and mastery, solidifying core ideas and preparing students for future lessons.
Skill Practice Questions
Students are tasked with answering practical, assessment-style questions such as:
- How many electrons can occupy a single orbital?
- What is the maximum number of electrons in an s, p, or d orbital?
- Fill orbital diagrams and write electron configurations for the first 20 elements.
Modifying This Electron Configuration Activity For Your Own Classroom
This electron configuration activity I’ve prepared is designed to be flexible, allowing you to tailor it to meet the needs of your students, no matter their level or learning style. Whether you’re working with students who are new to orbital diagrams or advanced learners ready to explore transition metals, this lesson can be customized to engage and challenge everyone.
Adjusting The Pace When Students Are Developing And Using Models
Time is, perhaps, the most important consideration to make when embarking upon guided-inquiry activities. When relying on exploration to lead student-centered learning, to recommend that you’ll need to allow for more time would be the understatement of the century! If you’re used to leading your class with lecture, this will feel very unnatural and even unnerving. Know that it doesn’t just take time for students to adapt to this kind of learning — there’s also a learning curve for you as you begin to morph into a servant leader!
On the other hand, advanced students (like those in honors-level courses or advanced prep schools) might move through this lesson more quickly and have the opportunity to begin developing and using models right away for other elements without the use of visual aids.
Normalizing Feelings Of Frustration
Some students might find the trial-and-error nature of developing and using models frustrating, especially if they’re used to the passive nature of a lecture-based lesson. But that’s exactly where the magic happens, here!
Be mindful to remind students that frustration is part of the scientific process. It’s a fact that scientists are more often wrong than they are right and that the learning happens from the hypotheses that are proven to be invalid!
Resist the urge to tell students what to do next! Simply offer encouragement and emphasize that lasting learning often is the result of mistakes we make along the way.
Strategically grouping students and connecting through collaboration in the face of adversity is also a great way to keep the tone positive and productive in your classroom.
Making These Models Work Across Your Curriculum
It’s possible to ditch lecture and lead active learning in your high school chemistry classroom each and everyday!
The models discussed and explored in this lesson are used throughout this unit all about electrons’ energy, location, and behavior.

A year-long curriculum of related lessons will act synergistically to magnify the benefits of employing an inquiry-based approach due to the consistency of experiences and opportunities you’ll be able to provide.
If this sounds like something you might like to explore, be sure to download free samples of more lessons from my micro2MACRO year-long chemistry curriculum!
Transform Your Teaching with Inquiry-Based Learning
This electron configuration activity demonstrates the power of inquiry-based learning to make science — at any level — interesting, engaging and accessible for every student. By uncovering key principles through student-controlled exploration and guided prompting, students gain a deeper understanding of the content while developing critical thinking skills that will serve them throughout their academic journey.