In today’s ever-evolving educational landscape, science teachers are tasked with integrating complex concepts into their lesson plans while fostering active learning environments. The Cross-cutting concepts (CCCs), outlined by the Next Generation Science Standards (NGSS), are one of the essential tools we have to help us accomplish this.
These seven concepts are central to all the science content we teach. By integrating them consistently in the lesson plans we design for active learning, we can condition students to think more critically, make connections across different scientific disciplines, and apply their knowledge in real-world scenarios.
But how do we, as educators, effectively integrate these concepts into our science teaching practice?
This guide will explore each cross-cutting concept in detail, providing practical lesson planning strategies and prompts to use during delivery that will not only engage your students but also reveal the core ideas of the content you teach in an inquiry-based or discovery-based way.
If you’d rather watch or listen, I’ve got you covered on YouTube and via podcast:
What Are The Cross-Cutting Concepts?
Cross-cutting concepts are the lenses through which students can view and understand the natural world.
They act as a bridge between different scientific disciplines, allowing students to transfer their learning from one context to another. The NGSS identifies seven key cross-cutting concepts:
- Patterns
- Cause and Effect
- Scale, Proportion, and Quantity
- Systems and System Models
- Energy and Matter
- Structure and Function
- Stability and Change
The NGSS also assigns at least one cross-cutting concept to each standard, along with core ideas and science and engineering practices.
However, each of these seven concepts can encourage students to develop critical thinking skills that are essential not only in science but in everyday life. They are useful for all of us beyond the structure defined by the NGSS!

By building in the opportunity for students to explore as many of these tenants as possible, we can transform traditional (and boring!) passive learning lectures into active exploration experiences, making science more memorable and more meaningful for your students.
Let’s consider each of the seven concepts closely.
1. Patterns, The Foundation of Scientific Inquiry

Understanding Patterns in Science
Patterns are fundamental to and abounding within science because they represent regularities and trends that can be observed in nature.
By recognizing patterns, students can make predictions, identify relationships, and develop models that explain natural phenomena. This concept is especially useful in the early stages of scientific inquiry, where observation is key.
How To Plan For Patterns
When planning science lessons where patterns might best reveal the core idea of your content, consider the following options:
- Lean On Observation Activities:
- Start with simple observation tasks where students identify patterns in data or natural systems. For example, in a biology class, students might observe the growth patterns of plants under different light conditions.
- Use Graphic Organizers For Observations:
- Prepare artifact outlines that allow students to more easily see the trend or pattern involved with the core content idea. This helps them to see the regularities more clearly and to communicate their findings effectively.
- Have Students Use Patterns To Make Predictions:
- Ask students to predict future outcomes based on observed patterns once they identify them. This not only reinforces their understanding of the pattern and core content idea they’ve unearthed, but also challenges them to think critically so that they can use that information to solve related problems.
In this example from my chemistry curriculum, each student uses a computer simulation to build stable and unstable isotopes of a single atom.
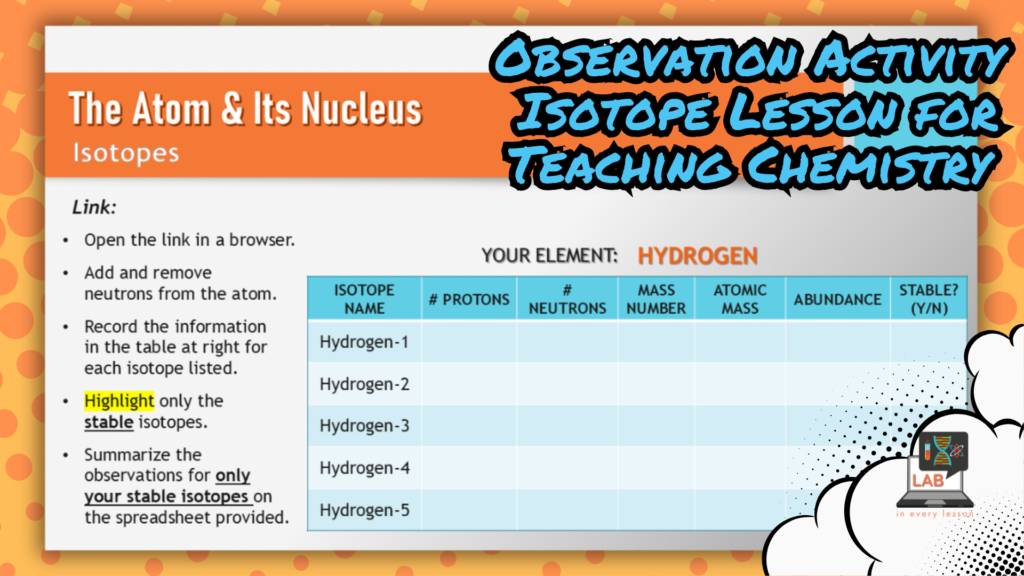
When all the students in a small group contribute their stable isotope data to a shared graphic organizer (setup in a simple Google spreadsheet), I’m able to ask specific questions that require them to identify patterns like how the number of protons in various isotopes of a single atom stays the same but all other properties change with the number of neutrons.
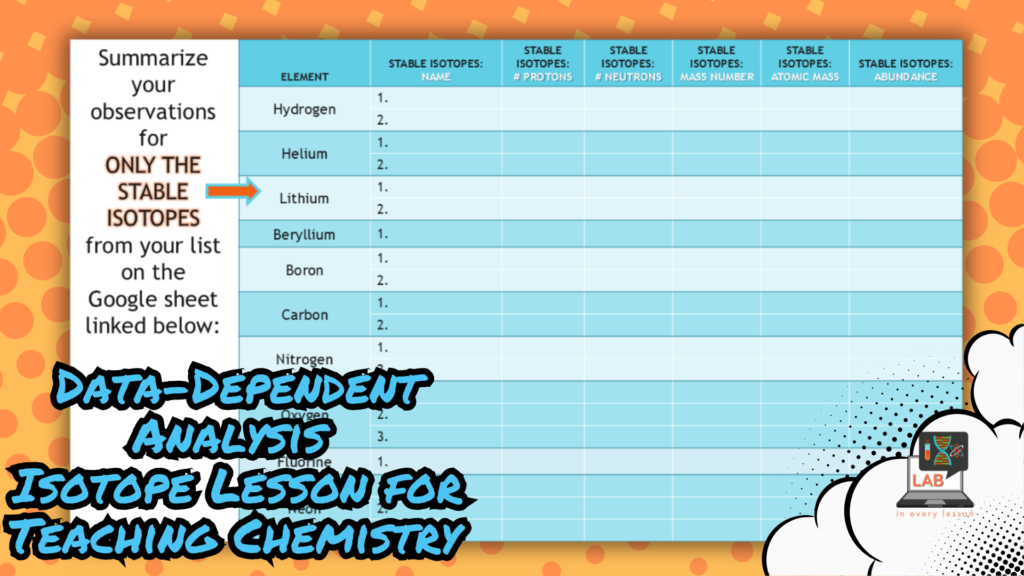
This question and answer segment of the lesson represents data-dependent analysis that is a critical step in the practice of science as we seek to learn and apply new information.
Pattern-Related Questions You Can Use
- What patterns do you observe in the data?
- Can you identify any regularities or recurring trends?
- How can we represent these patterns visually or mathematically?
- What predictions can we make based on the observed patterns?
2. Cause and Effect: Exploring Relationships in Science
The Importance of Cause and Effect
Cause and effect is a critical concept in science because it helps students understand the relationships between different events and phenomena.
By identifying causes and their effects, students can build a deeper understanding of how the world works, from the cellular level to the cosmic scale.

Practical Applications in the Classroom
To integrate cause and effect into your teaching, try these approaches:
- Ensure Students Control Outcomes:
- Have students control experiments in which they manipulate one variable to observe its effect on another. This can also be accomplished from video content when the cause is microscopic (or smaller!) and the effect is macroscopic.
- For example, in biology, students would not be able to observe directly the cause and effect relationship between genotypes and phenotypes.
- In chemistry, similarly, without a virtual simulation or animated option, students could not otherwise cite specific evidence to connect the degree of intermolecular attraction between molecules or atoms to the shapes adopted by specific states of matter (or lack thereof).
- Have students control experiments in which they manipulate one variable to observe its effect on another. This can also be accomplished from video content when the cause is microscopic (or smaller!) and the effect is macroscopic.
- Include Text-Based Case Studies:
- Use real-world examples and published articles to inform cause and effect. For instance, in an environmental science class, students might study the impact of pollution on local ecosystems.
- Prompt Discussion and Debate:
- Engage students in discussions where they explore alternative explanations for observed phenomena. This encourages them to consider multiple perspectives.
Cause and Effect Questions You Can Use
- What factors or variables are influencing the outcomes?
- How can we determine the relationships between phenomena?
- What evidence supports that this cause led to this effect?
- Are there any alternative explanations?
3. Scale, Proportion, and Quantity: Making Sense of Measurements
Why Scale, Proportion, and Quantity Matter
Understanding scale, proportion, and quantity is essential for students as they engage with scientific concepts that involve detailed measurements as in the physical sciences.
This concept helps students to grasp the relative size of objects, the importance of accurate measurements, and how changes in scale can impact the behavior of systems.
Strategies for Teaching Scale, Proportion, and Quantity
- Make and Use Meaningful Models:
- Many concepts within the science content we teach is intangible. Some of it is too small to measure directly, like atoms and cells, while some is too large to measure, like planets. In astronomy and planetary sciences, specifically, the distance of objects from our eyes makes our understanding of their size even more unlikely!
- Here, we can tap into technology so that our students can make quality comparisons to better discern the gravity of scale, proportion and quantity.
- Do The Math:
- Unit conversion makes it possible to better conceptualize extremes of size and quantity, while graphed data allows us to visualize proportions and size/quantity relationships. This is also an interdisciplinary task that reinforces math skills!
- Connect Cause and Effect:
- Sometimes, an object’s size enables its behavior or the change in quantity of an object determines an outcome. For example, atoms gain and lose electrons. This produces a non-neutral, charged atom which has a function unique and different from its neutral counterpart.

In this example from my chemistry curriculum, students use models to consider how an atom would gain or lose electrons to satisfy the octet rule, a natural phenomena that forms the foundation for most chemical change. They also work to determine the final charge on that atom. In a future lesson, they do this analysis for atoms within various groups of the periodic table to identify a pattern we call the periodic law.
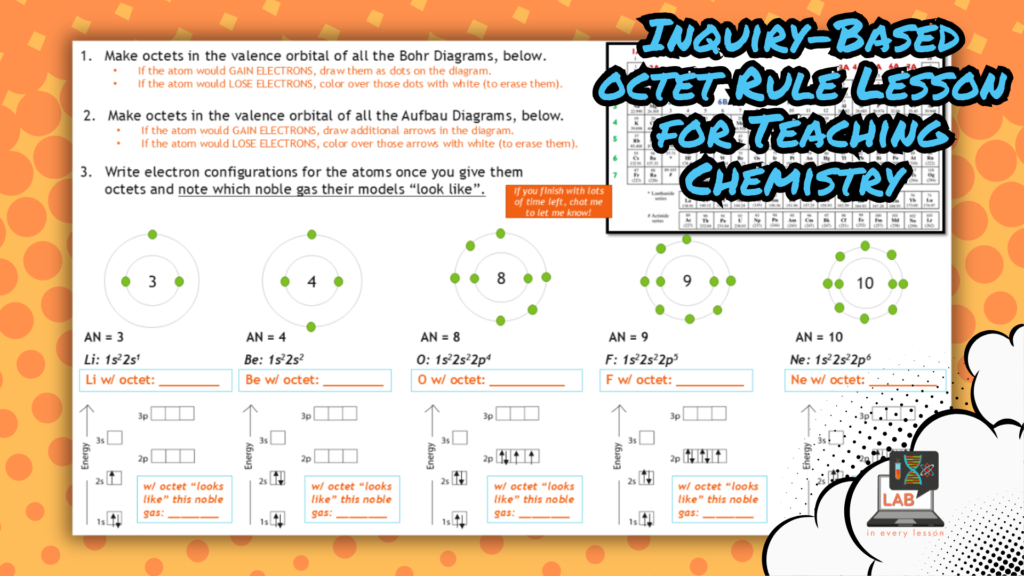
Scale, Proportion, and Quantity Questions You Can Use
- Can we use math or graphs to analyze the relationships between scale and quantity?
- How does the system change in response to changes in scale or proportion?
- What are the implications of different scales or proportions on the behavior of the system?
4. Systems and System Models: Understanding Complex Interactions
The Role of Systems and System Models in Science

Systems and system models help students understand how different components of a system interact with each other. Whether it’s the human body, an ecosystem, or an atom, this concept is critical for grasping the complexity of scientific phenomena.
Teaching Systems and System Models
- Make and Use Meaningful Models:
- Plan for students to manipulate models of systems they are studying. This could be achieved using a physical model, a puzzle-style diagram, or a computer simulation. Manipulating models helps students to identify and understand the components of the system rather than just simply memorizing them.
- Outline Investigations:
- In order for students to accurately analyze how different parts of a system interact and influence one another, they might need clear, systematic instructions. Don’t expect students to know exactly how to poke around a complex system if you don’t have the necessary time to spare!
- When cause and effect is also involved, your influence and insight into experimental design could be especially important.
In this example from my chemistry curriculum, students use a simulation to examine various orbitals within an atom and how electrons are able to move among them but not between them. With a thoughtful artifact outline design, students are able to also understand the role of energy in these processes.

While ‘energy and matter’ is considered a cross-cutting concept in and of itself (we’ll consider that one next), energy is a necessary component in the Bohr model of the atom. In fact, it’s more than a component — it’s the very reason electrons bounce around, absorbing and emitting energy, as they do! So, we’ve got cause and effect embodied in this activity also.
Taking it one step further, the graphic organizer I provide students to organize the data they collect enables them to identify energetic patterns that students can use to explain why various colors are emitted from the atom.
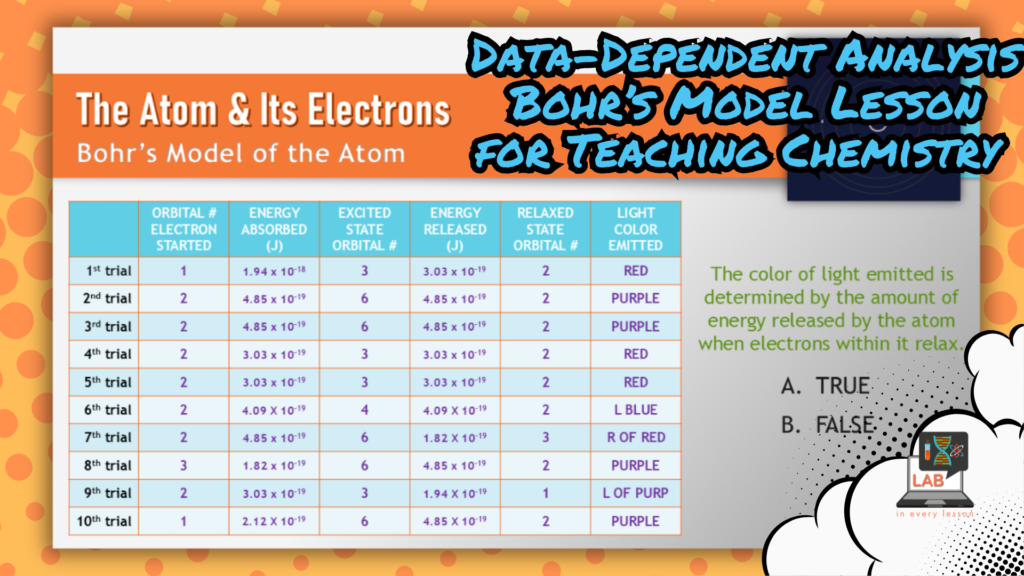
Systems and system models, cause and effect, patterns, and energy and matter … at least four cross-cutting concepts can be incorporated into a single learning experience! While you can focus on one to begin your activity designs, you might find that your final product includes many others.
System Model Questions You Can Use:
- What are the component parts of the system?
- How do these components interact and influence each other?
- How can we modify the system model to predict outcomes?
- What are the limitations of the current model?
5. Energy and Matter: Exploring the Flow in Science
Why Energy and Matter Are Fundamental Concepts
Energy and matter are foundational concepts in science that explain how systems operate. Understanding how energy is transferred, converted, and conserved, as well as how matter changes in response to energy, is crucial for students as they explore various scientific disciplines.

Incorporating Energy and Matter into Lessons
- Construct Energy Flow Diagrams:
- Use diagrams to help students visualize how energy flows through matter – sometimes transforming it – in a system.
- For example, in a lesson on photosynthesis, students could create a diagram showing how radiant energy from the sun is converted into chemical energy in plants.
- Use diagrams to help students visualize how energy flows through matter – sometimes transforming it – in a system.
- Provide First-Hand Experience:
- Conduct experiments that demonstrate the conservation of energy and matter.
- I use this virtual tutorial for students to observe how both the mass of reactants always remains equal to the mass of the products of a chemical reaction on both the sub-microscopic and macroscopic levels even when it doesn’t appear so to the naked eye.
- Conduct experiments that demonstrate the conservation of energy and matter.
- Incorporate Cross-Disciplinary Connections:
- Talk with a colleague to see where your areas of expertise overlap. For example, if you teach physics and your neighbor teaches earth science, collaborate on a lesson to include the energetic principles of physics that dominate changes in the behavior of matter like in volcanic eruptions or earthquakes.
Energy and Matter Questions You Can Use
- How is energy transferred or transformed within the system?
- What are the different forms of energy involved?
- How can we track the flow of matter or energy through the system?
- How does the conservation of energy or matter apply in this situation?
6. Structure and Function: Linking Form to Purpose
The Significance of Structure and Function in Science
The concept of structure and function is integral to understanding how the physical characteristics of an object or system determine its behavior and role. This idea is prevalent in biology, chemistry, and engineering, where the form of something is often directly related to its function.
Effective Teaching Methods
- Create Comparative Analysis:
- Have students compare and contrast different structures and their functions.
- One content-specific example that comes to mind is the difference between the structure and function of cells, prokaryotic and eukaryotic.
- Have students compare and contrast different structures and their functions.
- Include Engineering Challenges:
- Students struggle to design experiments and engineer solutions. Using gradual release principles of curriculum design, we could incorporate opportunities in our plans for students to make small tweaks to systems and observe outcomes which will lead to inferences about the role structure plays in the function of a system.
- In chemistry, Le Chatelier’s principle, equilibrium and the effect of concentration of reactant can be explored using a simple, reversible, acid-base titration experiment. Here, students can continue to add solutions and document the outcomes to discern when and why the indicator appears and disappears. And, students don’t need to know all the nuances of these complex principles to do this exploration! That’s precisely the point!
- Students struggle to design experiments and engineer solutions. Using gradual release principles of curriculum design, we could incorporate opportunities in our plans for students to make small tweaks to systems and observe outcomes which will lead to inferences about the role structure plays in the function of a system.
- Keep It Real With Examples:
- Connect core ideas to real-world scenarios, and do it while you strengthen disciplinary literacy skills.
- Use popular science articles and related text to ensure students are aware of how the systems they study are at work in their worlds.
- I’ve done this with airbags to help students contextualize combustion reactions beyond campfires, stoichiometry that takes place outside the kitchen, and gas properties that save lives.
- Connect core ideas to real-world scenarios, and do it while you strengthen disciplinary literacy skills.

Structure and Function Questions You Can Use:
- What are the specific structures or features of the object or system?
- How do these features produce the function?
- How can we modify the structure to improve its function?
- What are the properties and placements of the components that allow the system to function effectively?
7. Stability and Change: Navigating Dynamic Systems
Understanding Stability and Change
Stability and change are essential concepts for understanding how systems maintain equilibrium and how they respond to disturbances. This concept is crucial in any fields where systems are constantly in flux.

Incorporating Stability and Change into Your Curriculum
- Case Studies:
- Yet another opportunity to strengthen disciplinary literacy skills! Use case studies – detailed, non-fiction accounts of real-life scenarios – to illustrate how systems change over time.
- State universities often have extension programs that conduct and publish this type of research. Sharing their findings with students localizes the learning and could help students develop a greater appreciation for the phenomena they observe in and around their neighborhoods.
- Yet another opportunity to strengthen disciplinary literacy skills! Use case studies – detailed, non-fiction accounts of real-life scenarios – to illustrate how systems change over time.
- Plan for Predictions:
- Provide data for students to identify patterns and then use those patterns to anticipate the change that might occur if those patterns were to persist. In our modern society where, often, most things seem disposable, these kinds of activities might attach gravity to issues and resources that we often take for granted.
- Although it’s not my area of expertise, population dynamics in ecosystems come to mind!
- Provide data for students to identify patterns and then use those patterns to anticipate the change that might occur if those patterns were to persist. In our modern society where, often, most things seem disposable, these kinds of activities might attach gravity to issues and resources that we often take for granted.
Stability and Change Questions You Can Use:
- Is the system currently in a stable or balanced state? Why or why not?
- What factors can disrupt the stability of the system?
- How does the system respond to changes or disturbances?
- Can we predict the long-term behavior or stability of the system?
Need A Cheat Sheet?
Integrating cross-cutting concepts into your science curriculum is more than just meeting standards—it’s about equipping your students with the tools they need to think critically and make connections across different scientific disciplines. By designing activities that encourage active learning and delivering them with a student-centered approach, you can help your students develop a deeper understanding of science that will serve them well beyond the classroom.
This Cross-Cutting Concepts Cheat Sheet was designed as a handy guide to keep nearby during your planning AND to share with your students as they work through inquiry-based learning experiences.

Each one-page pdf includes a summary of the seven cross-cutting concepts described in this article and prompts to encourage their consideration.
Upon printing the 8.5″ x 11″ resource, you can cut down the middle and laminate them together for a front side / back side, bookmark style go-to guide!
It’s just one of the dozens of resources included in The Digital Instructional Design Studio. Whether you’re just beginning to implement the cross-cutting concepts in your curriculum or you’re looking to refine your lesson planning and delivery approach, some time spent in The Studio will surely equip you with tremendous tools for taking the next step toward more dynamic, engaging, and effective science instruction!
CLICK HERE to explore the possibilities!





