Teaching the Bohr model is often where the journey of understanding chemical change begins in high school chemistry. Using this Bohr model activity as part of a larger inquiry-based science lesson allows students to connect the microscopic world of electron transitions to macroscopic phenomena like flame colors, neon signs, and even fluorescence. By engaging students in observation-based learning, this activity encourages exploration of how electrons move between energy levels, emit light, and influence chemical reactivity.
Unlike traditional lecture-based lessons, this inquiry-based science lesson emphasizes student discovery and active engagement. Using simulations, graphic organizers for data collection, and guided prompts, students observe patterns, analyze data, and develop critical thinking skills. They uncover the cause-and-effect relationships that drive atomic behavior, gaining a deeper understanding of how energy transitions relate to phenomena like the flame test. Whether you’re introducing the concept for the first time or deepening student understanding, this activity bridges the gap between theoretical models and real-world applications.
Rather watch than read? I got you!
Check it out here:
Table of Contents
- How Inquiry-Based Learning Shapes the Bohr Model Activity
- Features of This Inquiry-Based Science Lesson
- Bringing the Bohr Model Activity to Life
- Adapting and Scaling the Bohr Model Activity
- Take the Next Step: Build Connections Across Your Curriculum
- Conclusion: Transform Your Teaching with Inquiry-Based Learning
How Inquiry-Based Learning Shapes the Bohr Model Activity
This lesson reflects my core philosophy: science should be active, engaging, and grounded in observation. Every inquiry-based science lesson I create is designed to meet students where they are, guiding them to explore phenomena, analyze evidence, and collaborate on their findings. With this Bohr model activity, students investigate the relationships between electron behavior, energy, and observable phenomena, building critical thinking skills along the way.
Through this approach, students aren’t just learning about Bohr’s model—they’re discovering how patterns and cause-and-effect relationships in atomic behavior connect to real-world applications like flame colors and neon signs. By emphasizing inquiry, this lesson transforms abstract concepts into meaningful, student-controlled learning experiences.
Features That Make This Bohr Model Activity a True Inquiry-Based Science Lesson
This Bohr model activity includes observation-based activities and opportunities for students to analyze both qualitative and quantitative data. It’s designed to highlight critical patterns within the periodic table and the behavior of electrons, helping students connect microscopic electron transitions to macroscopic phenomena like flame colors and neon signs.
By combining simulations with guided prompts and opportunities for argumentation from evidence, this lesson keeps students engaged while fostering critical thinking. Whether you’re introducing Bohr’s model for the first time or diving deeper into related concepts, this inquiry-based science lesson bridges the gap between theoretical understanding and real-world applications.
Bringing This Inquiry-Based Science Lesson to Life
Every piece of this Bohr model activity has been intentionally designed to engage students in discovery, connect them to real-world phenomena, and build their confidence as scientists. Let’s walk through how this lesson unfolds and how it ties everything together.
Start Strong: Connecting Concepts with the Bohr Model Activity Warm-Up
The lesson starts with a warm-up that revisits the timeline of atomic models. By grounding students in the history of scientific discovery, they’re reminded that every breakthrough builds on the work that came before it. At this stage, we review the foundational contributions of scientists like Thompson, Rutherford, and Chadwick, allowing students to reflect on what they already know about the atom.
This warm-up also sets the stage for introducing Niels Bohr. By focusing the discussion on his model, we begin to shift students’ thinking toward the relationships between electrons, energy, and reactivity. It’s like preparing the soil before planting seeds—students need this connection to move forward.

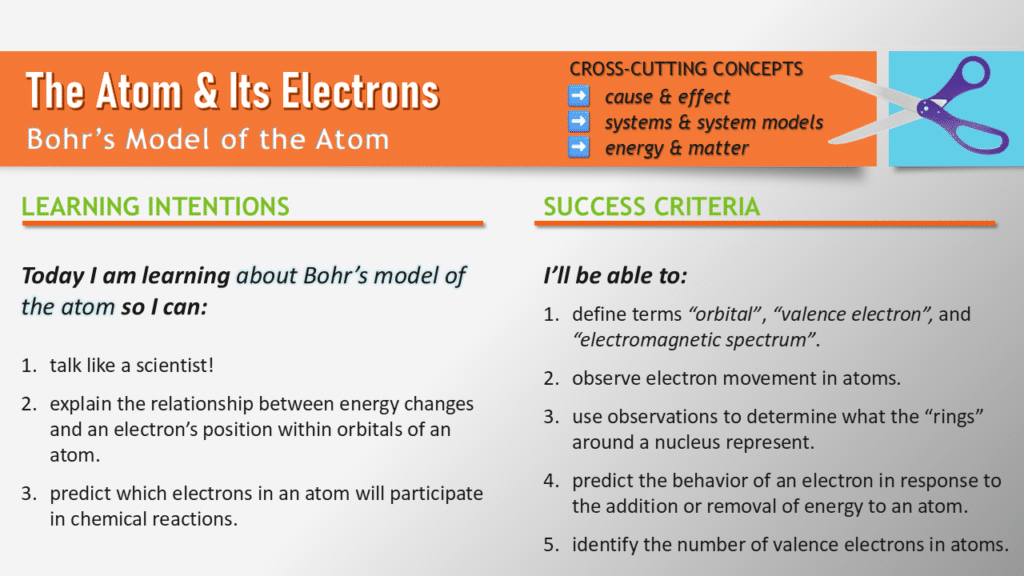
Guiding Discovery: Setting Objectives for an Inquiry-Based Science Lesson
Clear goals help students see where they’re headed, and this lesson’s objectives are no exception. We start by framing questions like, “Why are electrons never still?” and “Which electrons participate in chemical reactions?” These aren’t just abstract ideas—they’re the “why” behind the entire lesson.
One objective I’ve refined over the years is connecting energy changes to electron behavior. Originally, I phrased this as “understanding why electrons move.” Now, I align it more closely with three-dimensional standards by focusing on “explaining the relationship between energy changes and an electron’s position in an atom.” This shift makes the objective both more precise and more actionable for students.
Making Science student-controlled: Exploring Energy Through the Bohr Model Activity
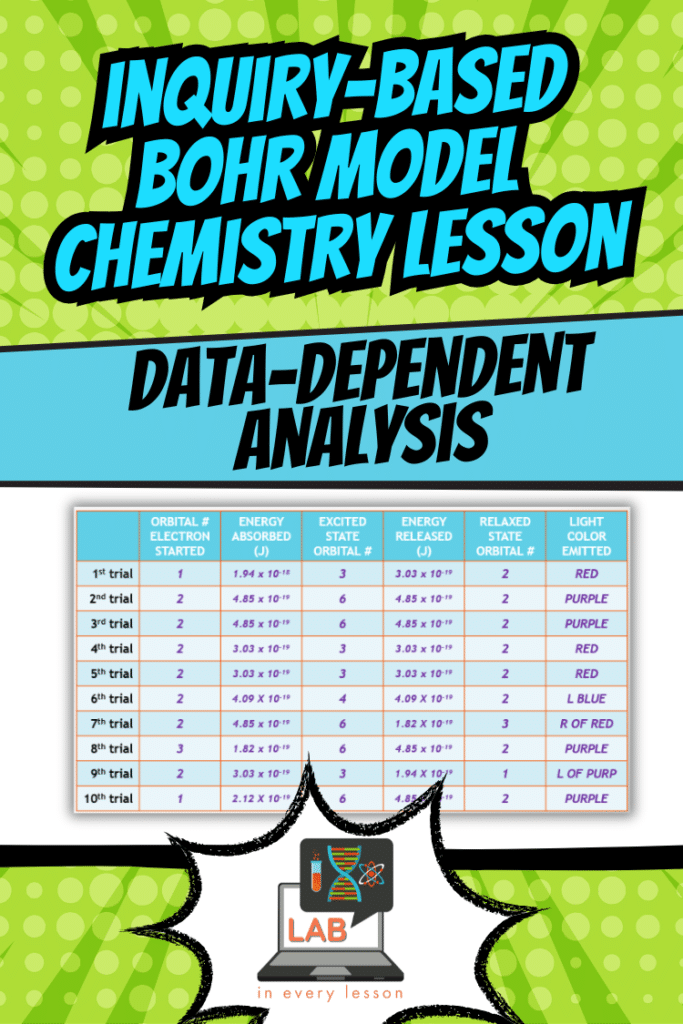
The real magic of this lesson happens during the simulation. This is where students get to uncover core ideas of the concept—without needing physical tools. They press a button, “zap” an atom with energy, and observe what happens. Each trial reveals how electrons absorb energy, move to higher orbitals, and release energy as they return to lower states.
What makes this simulation special is its ability to generate meaningful patterns for students to analyze. For instance, they might notice that certain energy transitions consistently produce specific colors of light. These connections help students link the microscopic (electron transitions) to the macroscopic (visible phenomena like flame colors or neon signs).
As a teacher, you can tailor this activity to your students’ needs. If you’re working with advanced students, they might record and analyze the data themselves. For others, you can provide pre-collected data to focus their attention on interpreting the results. Either way, the goal is to ensure every student walks away with a clear understanding of how energy drives electron behavior.
Documenting Learning: Artifacts for Inquiry-Based Science Success
To solidify their discoveries, students complete structured artifacts throughout the lesson. These aren’t just worksheets—they’re tools to help students document observations, synthesize ideas, and draw conclusions.
For example, one artifact guides students to reflect on how energy changes affect electron movement. Another introduces the concept of valence electrons, helping students connect these high-energy electrons to chemical reactivity. By the time students finish the activity, they have a robust set of notes and reflections that serve as evidence for their learning.
These artifacts aren’t just about recording facts—they’re about making connections. When students explain why certain electrons behave the way they do, they’re engaging in the kind of critical thinking that three-dimensional learning standards aim to develop.
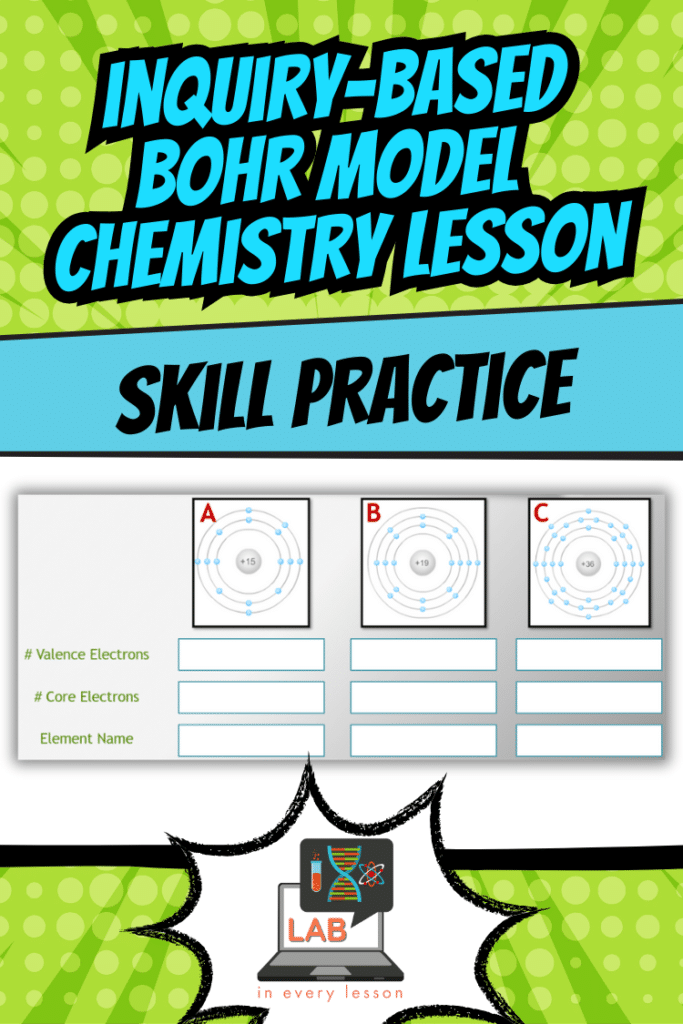
Skill Practice: Student Achievement on Bohr Model Assessments
The lesson wraps up with opportunities for skill practice, ensuring students can apply what they’ve learned. For instance, they might:
- Identify valence electrons in a given atom using the periodic table.
- Predict how an electron will behave when exposed to varying levels of energy.
- Explain why certain energy releases result in specific colors of light.
These tasks not only reinforce the lesson’s concepts but also prepare students for assessments. For example, you might show them a diagram of an atom and ask, “What happens when this electron absorbs energy?” Their ability to predict and explain the behavior demonstrates mastery of the material.
Exit tickets can also be a powerful way to gauge understanding. A simple question like, “Why do electrons emit light when they return to lower energy levels?” can reveal how well students grasp the underlying concepts.
Adapting and Scaling the Bohr Model Activity for Any Classroom
Every classroom is unique, and so is every group of students. This Bohr model activity has been designed with flexibility in mind, making it adaptable for various grade levels, learning styles, and teaching environments. Here’s how you can customize and extend the lesson while addressing potential challenges.
Customizing This Inquiry-Based Science Lesson for Different Grade Levels and Learning Speeds
One of the strengths of this lesson is its ability to scale based on the needs of your students. For advanced learners or upper-level chemistry classes, you might include data collection as part of the simulation. By having students record detailed observations and analyze patterns themselves, they can dig deeper into the quantitative aspects of energy transitions and electron behavior.
For younger students or those needing more scaffolding, you can simplify the simulation by providing pre-collected data. This approach allows students to focus on interpreting the results rather than the mechanics of gathering them. You can also adjust the depth of your guiding questions to match their prior knowledge, such as focusing on basic cause-and-effect relationships instead of advanced energy-level transitions.
Supplementary Activities to Deepen Learning
This activity doesn’t have to stand alone. It can be extended with supplementary lessons or mini-activities that reinforce core concepts. For example:
- Drawing Atomic Models: After completing the activity, students can sketch Bohr models for different elements. This hands-on practice helps them visualize valence electrons and their importance in chemical reactivity.
- Periodic Table Exploration: Use the patterns revealed in the activity to guide students in connecting electron configurations to periodic table trends, such as group and period numbers.
- Fluorescence and Beyond: Introduce the concept of fluorescence as a phenomenon tied to electron transitions. You could show a simple highlighter ink demonstration or discuss real-world applications like glow-in-the-dark materials.
These extensions provide an opportunity to link the Bohr model to broader scientific principles, making the lesson even more engaging and memorable.
How to Overcome Common Challenges with Inquiry-Based Science Lessons Like These
Even with a well-structured lesson, challenges can arise. Here are a few common issues teachers might face and strategies to overcome them:
- Time Constraints:
If you’re short on time, break the lesson into smaller segments. For instance, dedicate one period to the warm-up and simulation, and use the next period for discussion and analysis. This staggered approach ensures students can fully engage with each part of the lesson without feeling rushed. - Group Work Challenges:
Collaboration is a key focus of this activity, but group work can sometimes feel inefficient. To keep students on task, provide clear roles within each group—such as “data recorder,” “analyst,” and “discussion leader.” This structure helps streamline the process and ensures accountability. - Tech Accessibility:
If technology access is limited, consider running the simulation as a class demonstration rather than individually. Project the simulation and guide students through observations as a group, encouraging them to record notes and share ideas collaboratively.
Take the Next Step: Build Connections Across Your Curriculum
One of the most impactful reasons to use inquiry-based science lessons is how seamlessly they complement more cumulative content areas like chemistry. The Bohr model activity is designed not just as a standalone lesson but as a stepping stone to broader topics like periodic trends, ionization energy, and chemical reactivity.
To make the most of this lesson, consider how it might connect to your overall curriculum. Here are some practical ways to extend and integrate it into your teaching plan:
- Review and Preview with Warm-Ups: Use targeted warm-ups to revisit key ideas from this lesson—such as valence electrons and energy levels—and prepare students for upcoming topics like periodic trends or the octet rule. These short exercises help students spiral back to prior knowledge while staying engaged with the next step in their learning journey.
- Evidence-Based Drawing Tasks: Challenge students to draw atomic models that compare atom sizes or energy levels. Ask them to cite evidence for their conclusions, reinforcing both critical thinking and scientific communication skills.
If you’re looking for a cohesive way to streamline these transitions, my micro2MACRO Chemistry Curriculum offers the perfect solution. It’s designed to make lesson planning simple and flexible, allowing you to mix and match activities that align with your teaching goals. With resources that spiral back to previous lessons while introducing new concepts, this curriculum ensures every student can build on their understanding.
Ready to Get Started?
You don’t have to reinvent the wheel for every lesson. With my micro2MACRO Chemistry Curriculum, you’ll have a full suite of inquiry-based science lesson resources that work together seamlessly. Each lesson includes:
- Inquiry-based activities that encourage observation and critical thinking.
- Warm-ups and review materials to connect lessons and reinforce learning.
- Opportunities for differentiated instruction to meet your students where they are.
Plus, when you purchase the full curriculum, you gain access to all updates as they’re added—ensuring your resources stay fresh and relevant.

Bringing It All Together: Transform Your Teaching with Inquiry-Based Learning
This lesson isn’t just about teaching the Bohr model—it’s about transforming abstract ideas into meaningful, real-world applications. From the warm-up to the assessment, every element is designed to guide students through discovery, foster critical thinking, and build their confidence as independent learners.
For teachers, it offers a comprehensive, ready-to-implement framework that’s flexible enough to adapt to any classroom. Whether you’re introducing the Bohr model for the first time or looking to deepen student engagement with energy transitions and reactivity, this lesson provides the tools you need to bring learning to life.
Ready to Bring This Lesson to Your Classroom?
Take the next step toward creating dynamic, inquiry-based science experiences for your students:
- Click here to purchase the Bohr Model Activity as a standalone resource.
- Explore the full micro2MACRO Chemistry Curriculum, where this lesson is just one part of a complete system designed to save you time and seamlessly connect key chemistry concepts throughout the year.
- Learn how to plan activities and lessons like these with one of my professional development programs.
Looking Forward
Have you tried this lesson or integrated it into your teaching? I’d love to hear how it worked for you!
Share your experiences, ask questions, or connect with other teachers exploring inquiry-based science learning.
Let’s keep the conversation going as we inspire students to think critically and explore the world through science!





